
Loftware Cloud Compliance is the world’s first public, validation-ready Cloud labeling solution. It is the simplest way to design and print labels in a regulated environment.

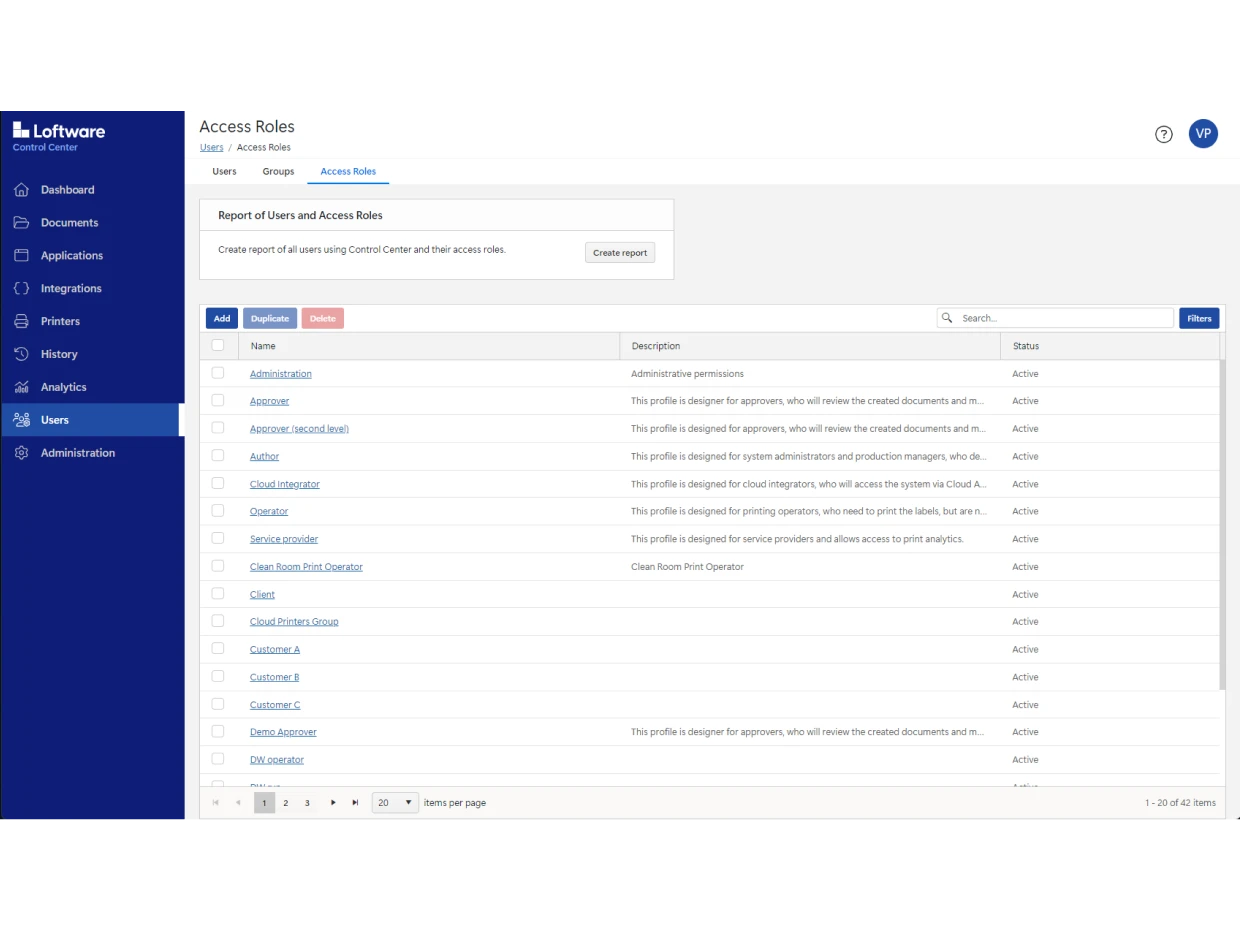
Use Loftware Cloud Compliance to digitize your entire approval process. You can also automate mass label changes and approvals without creating hundreds of label variations.
And you can test any changes before making them live in your production environment using the system’s three-tier environment: Development (DEV), Quality Assurance (QA) and Production (PROD).
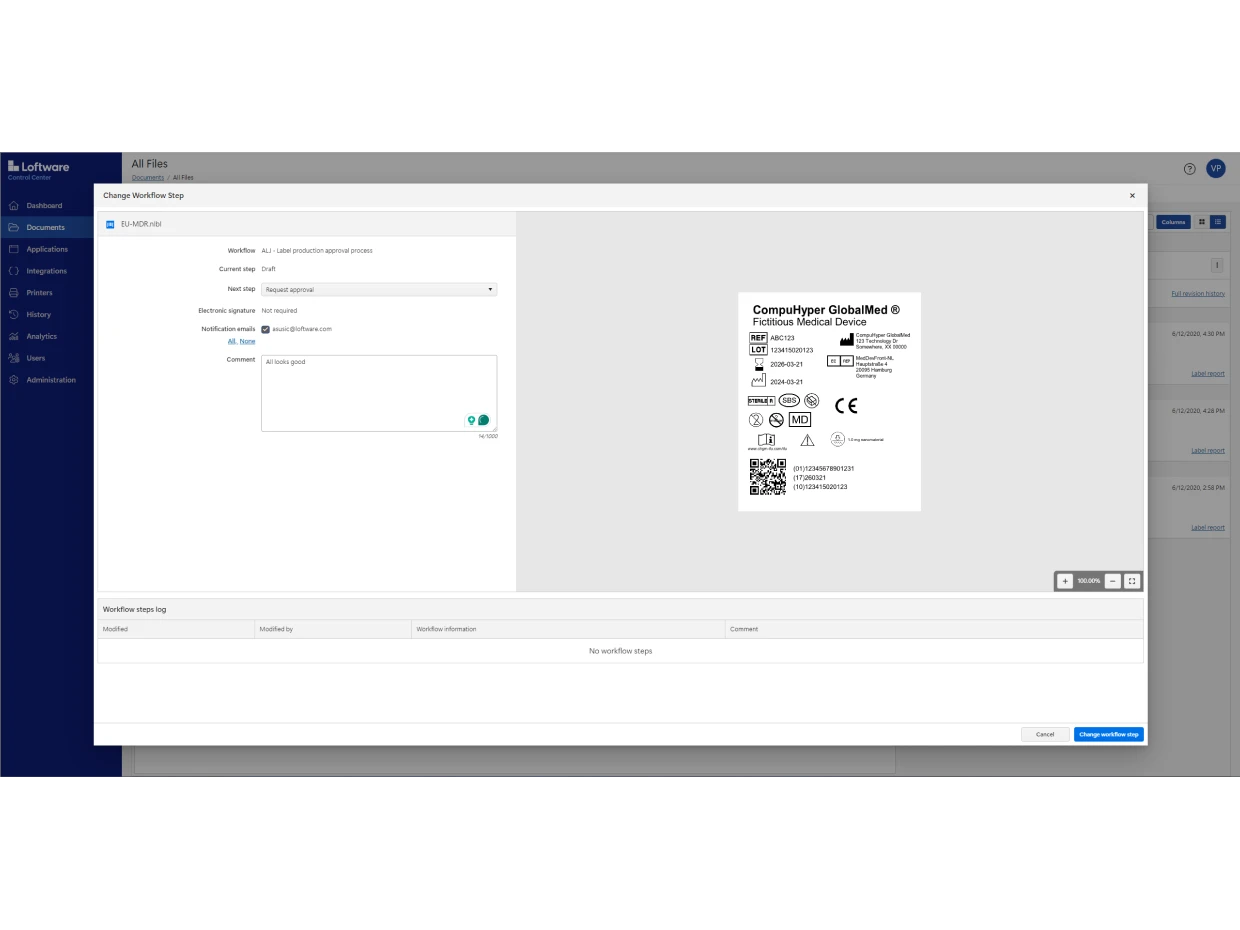
Loftware Cloud Compliance is designed to comply with the major regulations impacting the life science industry, including EU MDR, FDA UDI, FDA 21 CFR Part 11 and EU GMP Annex 11.
It includes built-in role-based access, document versioning, configurable approval workflows, electronic records and electronic signatures (ERES).
You also get a 12-year print history, which enables you to visually track every label you’ve printed during that time.

Loftware Cloud Compliance is a validation-ready labeling solution.
It is designed to streamline the validation process, which is further enhanced with the option of the Validation Acceleration Pack (VAP).
Our team can assist with IQ, OQ and PQ documentation as well. We also reduce the validation burden by only updating the software once a year. And when it’s time for the software release, we give you a three-month window before updating your production environment.